Drawing the Lewis Structure for SeF4
Viewing Notes:
Valence electrons are characteristics of metals. Selenium is a nonmetal. It accepts electrons, rather than releases them. The answer is II. Explanation: Going down the list that was provided, we can use the periodic table of elements to determine the valence electrons. Nitrogen has 5 valence electrons - Oxygen has 6 valence electrons - Fluorine has 7 valence electrons - Sulfur has 6 valence electrons - Selenium has 6 valence.
- In the SeF4 is Lewis structure Selenium (Se) is the least electronegative atom and goes in the center of the structure.
- SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons. Since there are seven Fluorine (F) atoms it will be necessary.
- You'll have a pair of electrons left over after filling octets of the F atoms. You can put these on the central Se atom.
- The Lewis structure for SeF4 has 34 valence electrons available to work with.
- It's a good idea to check the formal charges for your SeF4 Lewis structure to make sure they are zero.
See the Big List of Lewis Structures
Transcript: This is Dr. B. Let's do the Lewis structure for SeF4 Lewis Structure. Se on the periodic table is in Group Six so it has six valence electrons. Fluorine, seven valence electrons but we have four of those. So let's multiply that out ... six plus twenty eight equals thirty-four total valence electrons. We'll put the Se in the center and then the four Fluorine atoms on the outside, around it. So we've got thirty-four valence electrons. We'll put two between atoms to form the chemical bonds. So there's eight and then around atoms ... ten, twelve, fourteen, and thirty-two but I have thirty-four total.
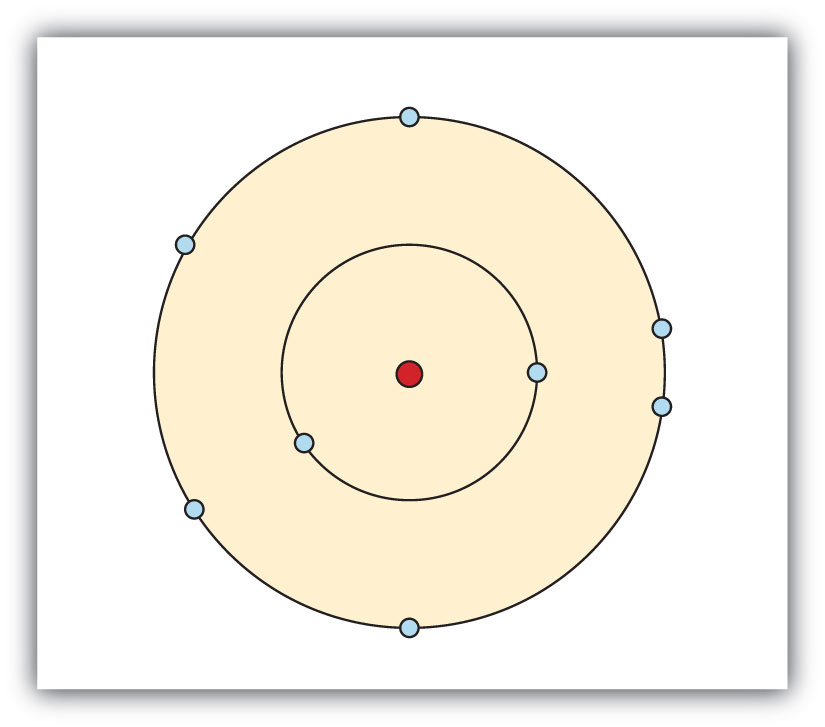
The number of electrons in per shell of selenium atom is 2, 8, 18, 6. So, there are six electrons in the valence shell of the selenium. The electronic configuration of selenium atom is 4s 2 3d 10 4p 4. Identify the nonmetal in period 4 that is more reactive than Selenium. Identify the element that has two valence electrons and three energy levels. Orbitals that would the valence electrons for selenium (Se) be placed is: s orbital and p orbital. Log in for more information.

Se Valence Electron Configuration
So I can't really add any more to the Fluorines. But since Selenium is in period three, we can put the remaining two right here on the central atom. so by putting these last two valence electrons on the center atom we've used up all my valance electrons. We should clean it up a little bit ... And we're done with the Lewis Structure for SeF4.
Element Selenium Valence Electrons
You could check the formal charges and if you did, you'd find out that they are zero for each one of those elements there making this the best Lewis structure for SeF4. This is Dr.B, and thanks for watching.
